Minerals
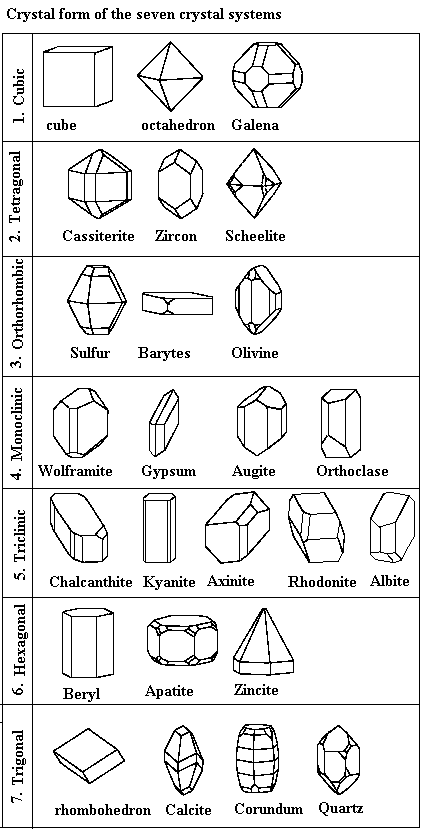 |
| Common crystal mineral crystal forms |
Minerals are the natural material that every inorganic material on planet Earth is composed of. They are the foundation of both our planet and most human industry. Minerals are defined as naturally occurring, inorganic solids that have characteristic chemical composition and crystalline structures. Because individual minerals must have the same chemical composition the atoms or molecules that make up each mineral exist in similar orderly three dimensional arrangements. The structure that results from such arrangement is called a crystal and is the external expression of the orderly arrangement of atoms and molecules.
In order for well formed crystals, as seen in the diagram to the left, to form the mineral must grow with little restriction. However due to competition for space among simultaneously forming crystals many minerals form inter-grown masses of mineral crystals.
Seven properties are commonly used to identify minerals: color, luster, hardness, streak, cleavage, fracture, and crystal form. For many of the minerals you will look at, these properties will be all that is necessary to identify the samples. However, at times other properties such as reaction to acid, magnetism, striation, and the existence of exsolution lamellae will be needed to make a correct identification.
Although color is the most obvious of mineral properties it is the least reliable for identification purposes. This is because the color of a mineral can be altered by slight chemical impurities within its crystal structure. For example, pure quartz is colorless but slight impurities can produce a wide range of color variety ranging from white to purple. In order to minimize the effect of impurities geologist depend on a mineral's streak to determine its true color. By definition the streak of a mineral is the color of a mineral in its powdered form. To obtain a mineral streak geologist rub the mineral on an abrasive ceramic tile called a streak plate.
The luster of a mineral describes the quality or appearance of light reflected from a mineral surface. Minerals can be divided into to major categories based on their luster; metallic lusters refer to minerals that have the appearance of metal (regardless of color) and nonmetallic lusters are minerals that tend to have more earthy appearances. Non metallic minerals are often described with adjectives such as glassy, pearly, silky, and earthy.
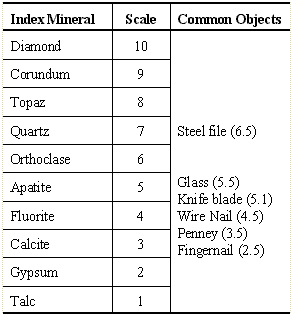 |
| Moh's Hardness Scale |
The ability of some minerals to scratch the streak plate while others may be scratched by the
streak plate is a result of another property known as hardness. The hardness of a mineral is a measurement of the strength of the chemical bonds between the individual atoms or molecules within the mineral. The strength of the bonds depends solely on the physical properties of the atoms within the bond. With over 2000 known minerals, each of which has its own definite chemical composition, using hardness as an identifying property could be a daunting task. This process is simplified by using a relative scale such as Mohs Scale of Hardness.To use this technique, hardness values between 1 and 10 have been assigned to some common minerals. 1, the softest, is a mineral known as talc; the hardest mineral, 10 on Mohs scale, is diamond. Along with assigning hardness values to common minerals, these values have also been assigned to other common objects as illustrated in the chart to the right.
Along with determining the hardness, the bonding and molecular structure of a given mineral will also determine the manner in which a mineral breaks. Surfaces held together by relatively weak bonds, such as those between repeated parallel layers of a crystal, will tend to break more easily than those held together by strong bonds. The tendency of a mineral to break along these flat parallel surfaces is known as cleavage.
The number and strength of bonds between the silica, aluminum, and hydroxide ions make those layers much stronger. Therefore, muscovite mica is much more likely to break along the layers that only contain the weakly bonded potassium ions. This results in 1 excellent cleavage plane of mica. This cleavage is observed in the ability of mica to peel into sheets.
Cleavage can be described as being excellent, good, poor, or absent. Excellent cleavage, such as that of muscovite mica, will result in smooth, flat, parallel surfaces. Good cleavage will often result in small, smooth, step-like flat surfaces. In minerals that exhibit poor cleavage, it may be difficult to identify any cleavage surfaces. In poor cleavage, the cleavage surface is often mixed in with fractured surfaces. If a mineral exhibits no cleavage, cleavage is said to be absent; in these cases the mineral is said to have fracture.
Fracture can be described as either being irregular or conchoidal. Conchoidal fracture will result in the mineral breaking along smooth, curved surfaces. Typically, these surfaces resemble the inside of clam shells.
