Mineral Groups
Silicates
| Silica Tetrahedra-the building block of silicates. |
Silicates make up over 95% of the Earth's crust and mantle, as such they are the most common minerals on Earth. Silicate are minerals that contain the fundamental SiO4 anion known as the silica tetrahedra. An anion is atomic structure that has a net negative charge a tetrahedra is a geometric shape similar to a four-sided pyramid. The silica tetrahedra is composed of one silica atom surounded by four oxygen atoms.
Since all minerals are neutral in charge, silicate minerals must include other atomic/molecular elements that balance or neutralize the negative charge of the SiO44-anion. How these atoms and tetrahedra come together determine the physical properties of the resulting structure, and therefore, the specific mineral that is formed. Silicate minerals are categorized based on the arrangement of silica tetrahedra and other atomic components.
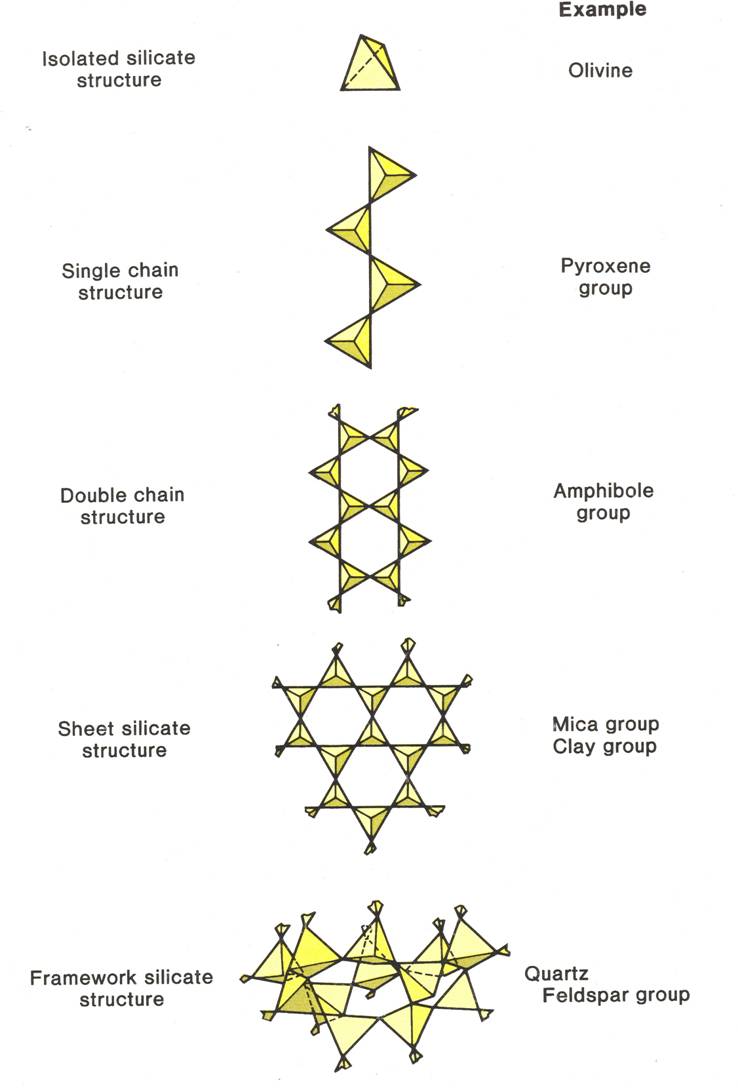 |
Minerals of the isolated tetrahedra group have crystal structures where no oxygen is shared between tetrahedra. In this group the mineral is held together by the attraction between silica tetrahedra and other positive ions. Olivine and Garnet are examples of minerals within the independent tetrahedra group.
Single chain silicates contain silica tetrahedra that are linked up in a chain by sharing two oxygen atoms. The most common single chain silicate is pyroxene.
Double chain silicates form by sharing either two or three oxygen. This group contains the mineral amphibole or hornblende
Sheet Silicates share three oxygens of the silica tetrahedra and form two-dimension sheets of silica. Other ions or even water molecules fit between the sheets to bond individual sheets together. Because of their structure sheet silicates tend to have excellent cleavage in one plane. Muscovite mica is an example of a sheet silicate
Framework Silicates are formed when all four oxygens of a silica tetrahedra are shared with adjacent tetrahedra to produce a three-dimensional network of tetrahedra. The most common framework silicates are potassium feldspar and plagioclase feldspar.
Other Mineral Groups
Oxides: Oxides are minerals that consist of metal cations (atoms with a positive charge) bonded to oxygen anions. Typical oxides include hematite (Fe2O3) and magnetite (Fe3O4).
Sulfides: Sulfides consist of metal cations bonding to a sulfide anion such as in the minerals pyrite (FeS2) and galena (PbS2)
Sulfate: Sulfates are minerals that consist of metal cations bonded to a sulfate (SO42-) anion.
Halides: Halides are mineral that consist of metal cations bonded to halide anions. Halide referes to the elements chlorine, flourine, iodine. Flourite is an example of a halide
Carbonates: Carbonates are minerals that contain metal cations bonded to the Carbonate (CO32-) anion. Calcite (CaCO3) is an example of a carbonate
Native Elements: Minerals that consist of one single element belong to the group of native elements. Examples of these minerals include copper, gold, and silver.
